Our foundation: People, experience, technology and capital
Reliability, honesty and diligence, and the ability to react quickly and in an uncomplicated manner, all of this with enthusiasm and passion – that is what we seek as a team, and that is what drives us. Our customers can rely on our competent and convenient service.
We know the markets and the products, and we feel we know what our clients need and demand when it comes to service, products and technology. We listen to our customers, and we don’t promise anything that we can’t deliver!
OBERON Fiber Technologies focuses on the two application areas in photonics: medicine & life sciences and industry & science. We develop and produce special fiber optic components for such areas as laser surgery medicine, medical diagnostics as well as for optical measurement technology in the area of sensory technology and analytics.
Our experience is your advantage
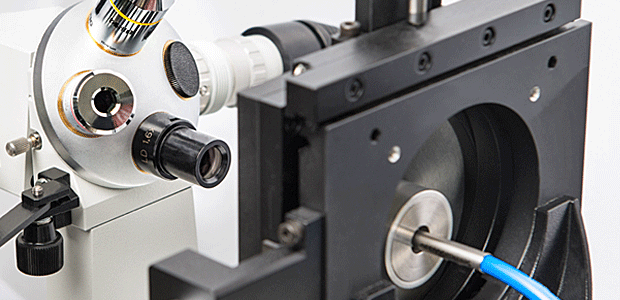
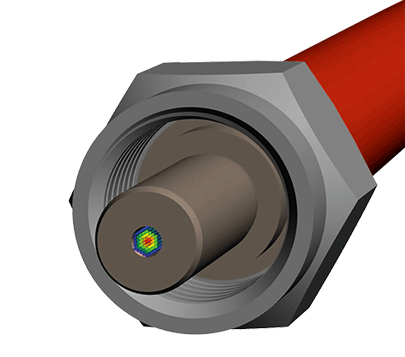
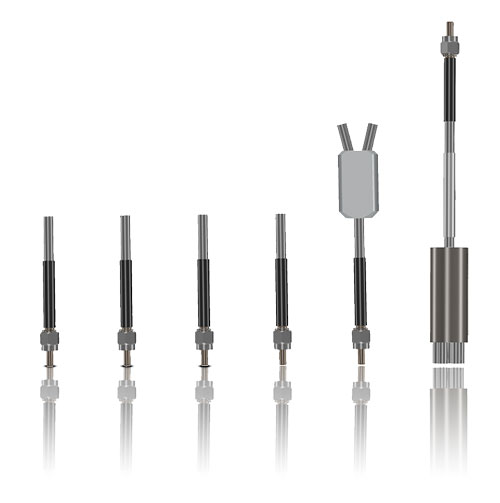
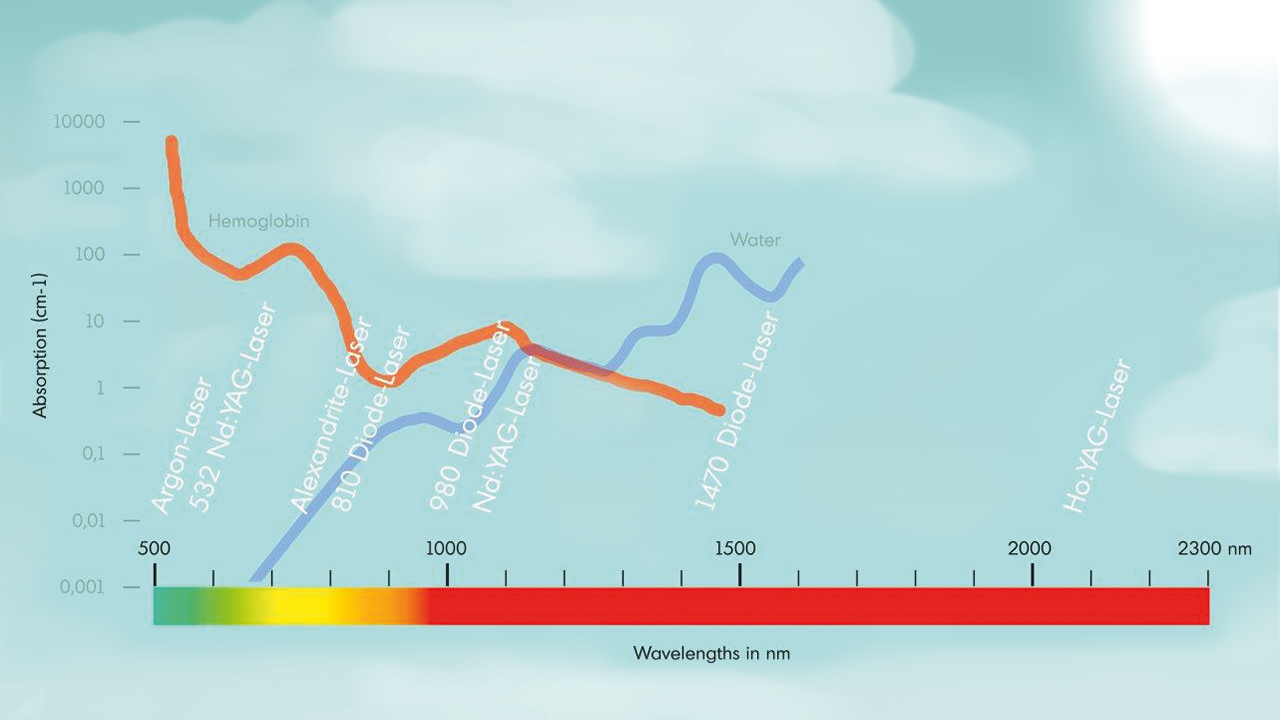
The basis of optical laser and measurement systems is the transmission of photons using our product, special system components with internal silica fibers. Our product portfolio includes fiber optical probes and special assemblies for transmitting visible and invisible light. In development and manufacturing, we distinguish between two technological applications: power transmission (laser beam delivery) and measurement data logging and transmission. One example of this kind of application is spectroscopy.
OBERON Fiber Technologies is a company with an experienced team. Every one of our company’s employees, whether in production, quality control, sales or product design, has over ten years of experience in his or her field.
OBERON Fiber Technologies operates with a minimum level of hierarchy, a minimum level of overhead and a maximum level of cost savings, which we pass on to our clients.
Triple safety standards for our product
OBERON Fiber Technologies takes quality seriously – quality that forms the basis for safety. Safety in the process, safety for the user, safety for the patient.
We demand reproducible quality of ourselves, and our clients demand it of us. That is why our quality management system conforms to EN ISO 9001 requirements.
OBERON Fiber Technologies meets the prerequisites to manufacture medical devices and bring them to market. We have carried out a conformity evaluation procedure in accordance with Annex II, Article 3 of Directive 93/42/EWG and implemented a comprehensive quality control system. As a result, we are licensed to give our medical devices a CE marking. We have initiated the supplemental quality management system in compliance with EN ISO 13485 requirements.
Medical product safety
OBERON Fiber Technologies has its medical Laser Surgery Fiber licensed with the USFDA under 510k no. K140470.
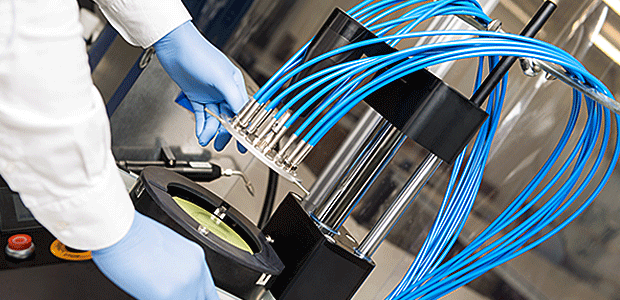
OBERON Fiber Technologies has sterility certifications in accordance with EN ISO 11135-1, endotoxin-free certifications, EO and ECH residual analyses and Bioburden tests for the medical devices we manufacture. The requirements under EN ISO 10993-1 on biocompatibility are met. The EtO sterility process is validated. All materials used for the packaging of our medical devices meet DIN EN ISO 11607 and DIN EN 868 requirements. We achieve reproducible environmental conditions in our ISO class-7 clean room. Clean room monitoring is regularly carried out by an accredited laboratory.
Our location
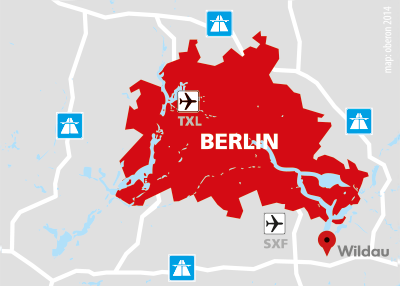
OBERON Fiber Technologies is based in the City of Wildau, to the southeast of Berlin. In the Aerospace Technology Center (www.zlur.de), we have 580 m² of production and development space with a clean room for class 7 medical device manufacturing.
We are located two kilometers from the Berlin autobahn ring, six kilometers from the Berlin city limits and eight kilometers from Schönefeld Airport. Our cooperation partner, the Technical University of Applied Sciences Wildau (www.th-wildau.de), with its Master’s degree program in photonics, is one kilometer away.
D - 15745 Wildau
Freiheitstr. 120
Entrance B (Sales & Engineering)
Entrance C (Production & Logistic)